How to track clinical trials and research studies

Why track and follow clinical trials?
Clinical trials play a crucial role in advancing medical research. They involve various stakeholders driven by diverse personal and professional motives.
-
Patients and healthcare professionals follow trials to participate in treatments and track the progress of a particular study.
-
Investors and sponsors track clinical trials for competitive intelligence and to ensure the effective use of resources. They keep tabs on review reports to check if the trial is on track to achieve it’s objectives. This helps them decide on continuing investments and potential commercialization opportunities.
-
Regulators track clinical trials to monitor the trial’s status and flag any deviations from compliance standards, reporting them as medical malpractice cases.
-
You may be interested in tracking clinical trials if you want to contribute to medical research and receive compensation or if you simply want to stay informed about the latest developments in clinical research studies.
All clinical trial announcements and study progress are posted on websites, making web monitoring critical for data surveillance. This involves tracking and analyzing information posted on online clinical trial registries for data like- participant recruitment, study protocols, adverse events, and study results.
If you fit into any of the categories above, we’ll help you set up alerts for the clinical trials you want to track on online registries. In this guide, we will demonstrate different ways to monitor clinical trials using a simple browser extension. You can find the different use-case for tracking clinical trials under this section.
Before we delve into the details, let’s provide you with a clear overview of the topics we’ll be covering. Below is a table of contents to help you navigate through the article:
- Where to track clinical trials
- How to find and track clinical trials
- How to use the filter options on the clinicaltrials.gov
- Exploring use cases: Different ways to track clinical trials with Distill
- Installing Distill web monitor
- Multiple ways you can set up Distill to monitor clinical trials
- How to track new studies announced for a particular clinical condition
- How to monitor the RSS feed on clinicaltrials.go
- How to track clinical trials backed by a particular sponsor
- How to track the recruitment status of a clinical trial
- How to track updates on the trial and check if study results are posted
- Steps to import and edit JSON
- Conclusion
Where to track clinical trials?
Below are a few trusted websites maintained by the National Institutes of Health (NIH), a credible and well-respected body in the field of medical research. The U.S. Food and Drug Administration (FDA) regulates the registry and results database of these websites as per federal regulations.
ClinicalTrials.gov: This is a publicly available database of clinical trials conducted in the United States and worldwide. It is managed by the National Institutes of Health (NIH) and is one of the largest and most comprehensive databases of clinical trials available.
NIH Clinical Center: NIH is the primary federal agency for conducting and funding biomedical and clinical research in the US. Their website also provides information on participating in the clinical studies at the NIH Clinical Center.
Research Match: This is a free online tool that helps researchers connect with individuals who are interested in participating in clinical trials. It also allows you to find clinical trials by aggregating trials registered on clinicaltrials.gov looking for volunteers for over two thousand medical conditions.
How to find and track clinical trials
With over 360,000 clinical trials registered on clinicaltrials.gov across 200 countries (as on September 2021), the number of trials has been growing annually by over 6%. This increase makes it challenging to keep track of all activities. Monitoring such a vast number of clinical trials can be overwhelming due to:
-
Limited volunteer slots, meaning patients may miss out on opportunities to participate in clinical trials.
-
The likelihood of missing important updates such as reports, publications, and deadline changes without proper tools and trackers. For regulators, manually tracking results can be highly time-consuming.
Missing updates to clinical trials may prove to be costly. We recommend using a website change monitoring tool to automate the process of tracking any online clinical trial registry. These are simple browser extensions that help:
-
Automate tracking of any number of clinical trials on any type of public clinical trial registry.
-
Trigger instant change notifications so you don’t miss any important updates in the clinical trial registry.
-
Track any parameter on the page like trial status, research schedule, announcements of new research studies, etc.
-
Organize and monitor multiple trial web pages across multiple clinical trial websites via a single dashboard.
These services are free and very easy to use. To track more web pages, you may have to explore paid plans. We will use the Distill browser extension to demonstrate how to track clinical trials online. Read ahead for step-by-step instructions or follow this video tutorial to track clinical trials.
How to use the filter options on the clinicaltrials.gov
You can follow the below steps to filter the clinical trials based on your requirements:
Step 1: Navigate to the clinicaltrials.gov.
Step 2: Click Advanced Search.
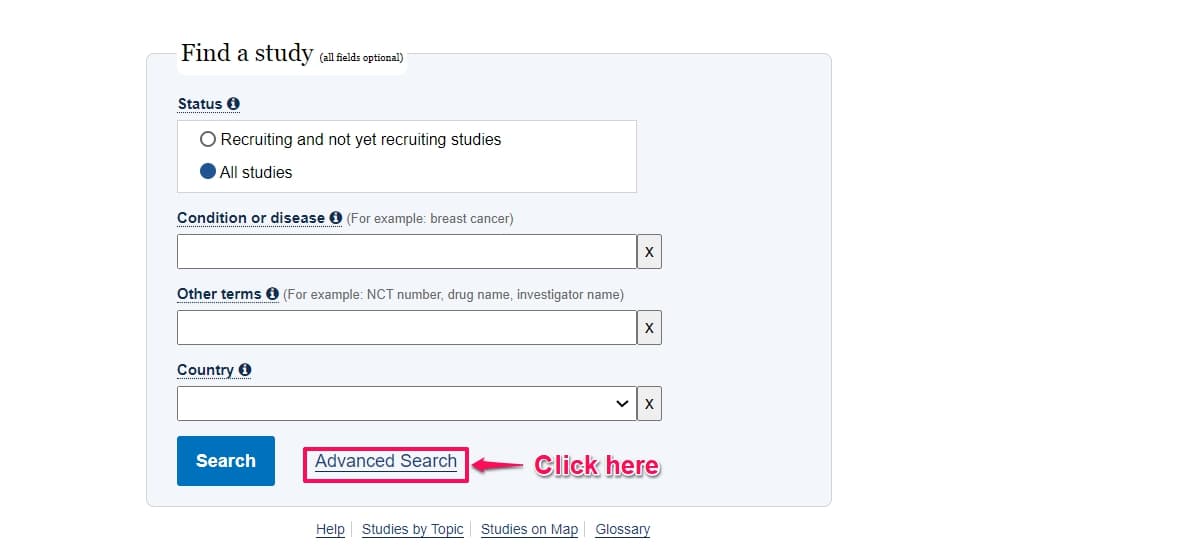
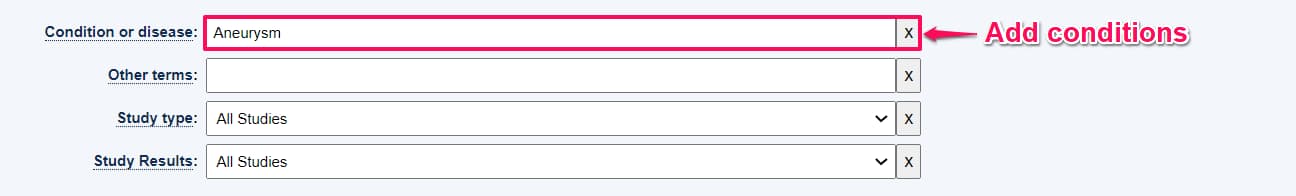
Step 3: Under conditions, type the medical condition you are looking for. For this example we will be looking for trials on Aneurysm.
Step 4: Under location type the country and state. This is primarily applicable in cases where patients are looking for volunteering opportunities within a specific city. For this example, we will look for trials on Aneurysm within a 100 miles radius of Miami, Florida.
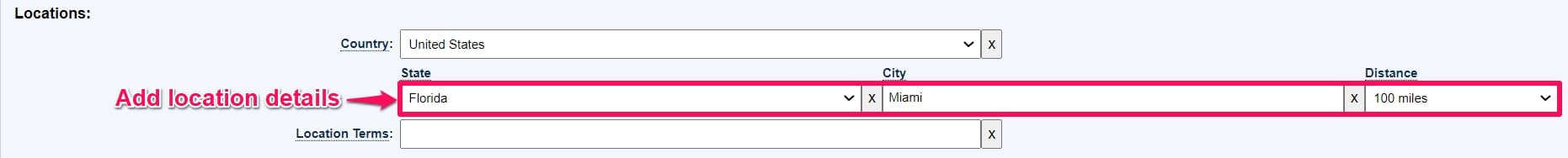
You can also filter the trials based on Recruitment status, age, sponsor, Phase, etc.
Step 5: Select Newest first under the sort studies option. This ensures clinical trials are listed from the most recent to the oldest record based on the First posted date.
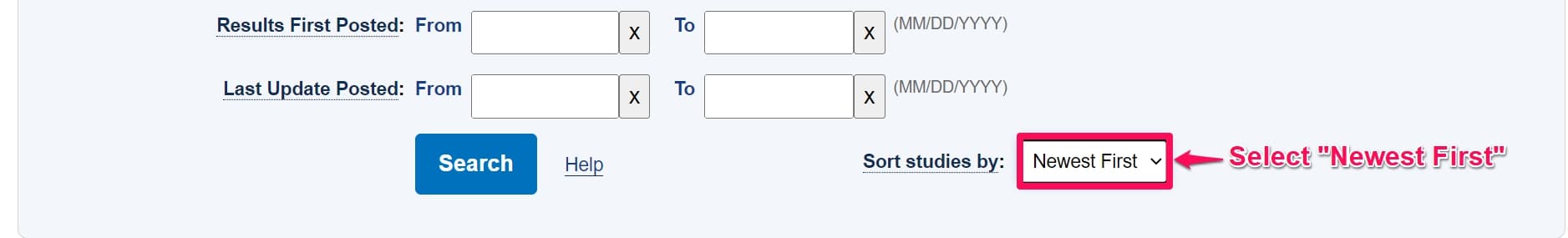
Step 6: Click Search.
Now that you are equipped to use the advanced filtering options on clinicaltrials.gov you can easily get trial feeds related to your requirements. The next steps would be to track these feeds or individual trial pages for updates. We’ll be using Distill web monitor to track the registry, read ahead to learn more.
Exploring use cases: Different ways to track clinical trials with Distill
Distill is a website change monitoring tool that allows you to configure it to track any section of a webpage with just a single click. After setting the frequency of tracking, it will send notifications if there are any changes in the selected section.
You can download Distill and begin using to track clinical trials of your interest. Let’s walk through how you can get started.
Installing Distill web monitor
Install Distill on a browser of your choice: Chrome, Firefox, Microsoft Edge, and Opera.
Optional: To pin the Distill extension, click the extension icon at the top right of your browser and click the pin icon next to Distill.
Multiple ways you can set up Distill to monitor clinical trials
Now, let’s explore how to set up Distill to track clinical trial registries. You can expand the use-cases that are of interest to you and follow along the step-by-step instructions to set up your monitor. Alternatively, you can also download the respective JSON files and import the ready-to-use monitors provided under every use-case.
How to track new studies announced for a particular clinical condition
You might want to track new clinical trials if you are:
-
A patient with a medical condition is not responding to current treatments and is interested in participating in a clinical trial to access new treatments or therapies.
-
A researcher who wants to track and follow new clinical trials in your field of study.
-
Someone who wants to contribute to medical research and be compensated for their time and efforts.
With Distill extension, you can monitor an entire feed under a particular study area in a specific location. Follow the below steps to set it up:
Step 1: Navigate to clinicaltrial.gov and filter the specific conditions you are looking for in a clinical trial and location. For this example, we will be tracking the clinical trials with the condition Lung Cancer in Florida, United States.
Step 2: Click the Distill icon on the top right corner of the webpage and select Monitor parts of page.
Step 3: To select the first record in the feed, click the serial number 1 and then click the caret icon and select expand selection. You will notice that the entire first row is now selected. This will ensure you get an alert every time the feed is updated with a new trial record.
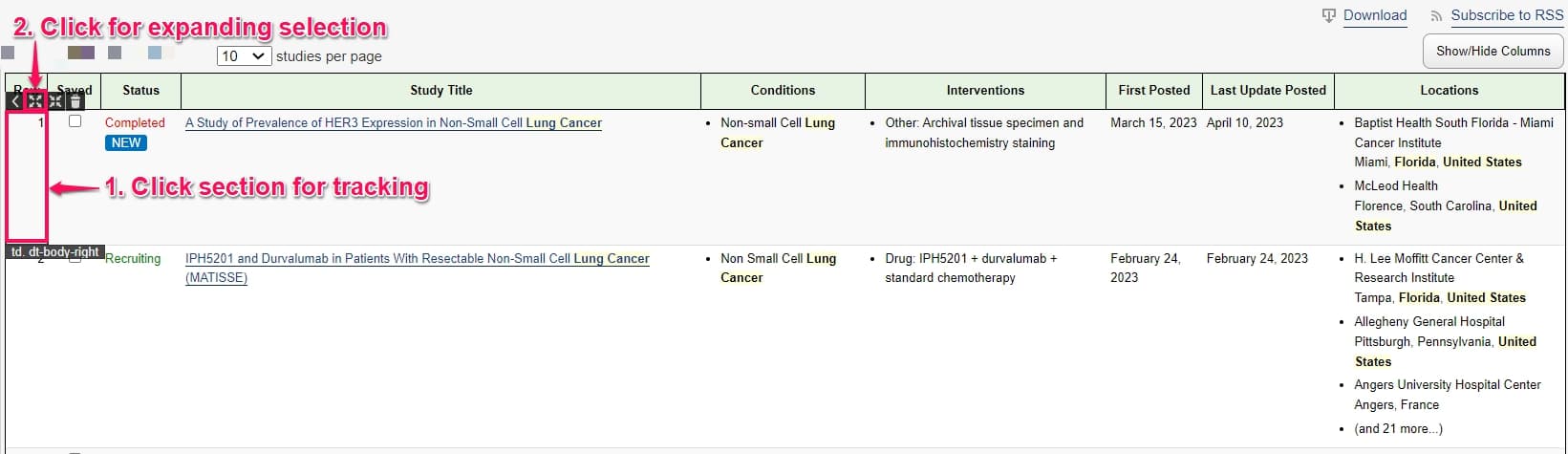
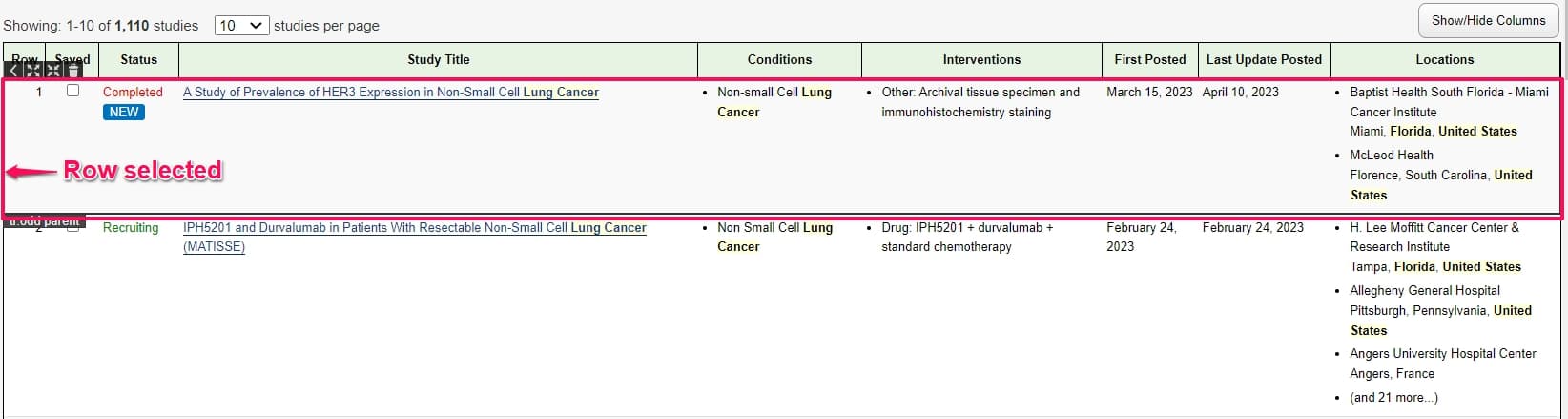
Note: Make sure you have sorted the clinical trials by Newest before setting up the monitor. For more details on how to do this, refer to this section.
Step 4: Click Save selections.
Step 5: You will be directed to the options page, where you can choose a device to run your monitor. We suggest using your local browser if you are using the free plan.
Step 6: Configure your alert mode via email/SMS/ Discord etc. Check out the subscription plans to compare features that suit your tracking requirement.
Step 7: Click Save.
Alternatively, you can follow this video tutorial to learn how to track new studies on clinicaltrials.gov.
How the monitor works:
Since the clinical studies are sorted by Newest first, anytime a new research study comes up, it will replace the content in row 1. This change is picked up by Distill, which will alert you that a new study has come up on the feed.
You can follow the steps given in this section to import and edit the JSON provided below.
Here is the JSON to track new studies announced for Lung Cancer in Florida, United States: Download Monitor JSON
How to monitor the RSS feed on clinicaltrials.gov
You can apply filters you need to modify your feed to reflect only the data of clinical trials you want to track. You can read more about how to set up an RSS feed for a specific search on clinicaltrials.gov. The RSS feed will automatically list additions and updates to your search results.
Follow the below steps or this video tutorial to set up an RSS feed monitor for a specific filter. You can begin applying filters by landing on clinicaltrials.gov:
Step 1: Navigate to the clinical trial registry. For this use case, we will use the same example mentioned above. We’ll set up an RSS monitor for tracking new studies that have been announced for Lung Cancer in Florida, United States.
Step 2: Click Subscribe to RSS.

Step 3: Select a feed type. We will select the option Show studies added or modified (last update posted) in the last 14 days for this example.
Step 4: Click Create RSS Feed. (Refrence: URL of RSS feed for this example).
Step 5: Copy the URL of the web page generated for the RSS Feed. The RSS feed typically contains the word rss or feed and ends with the file extension .xml.
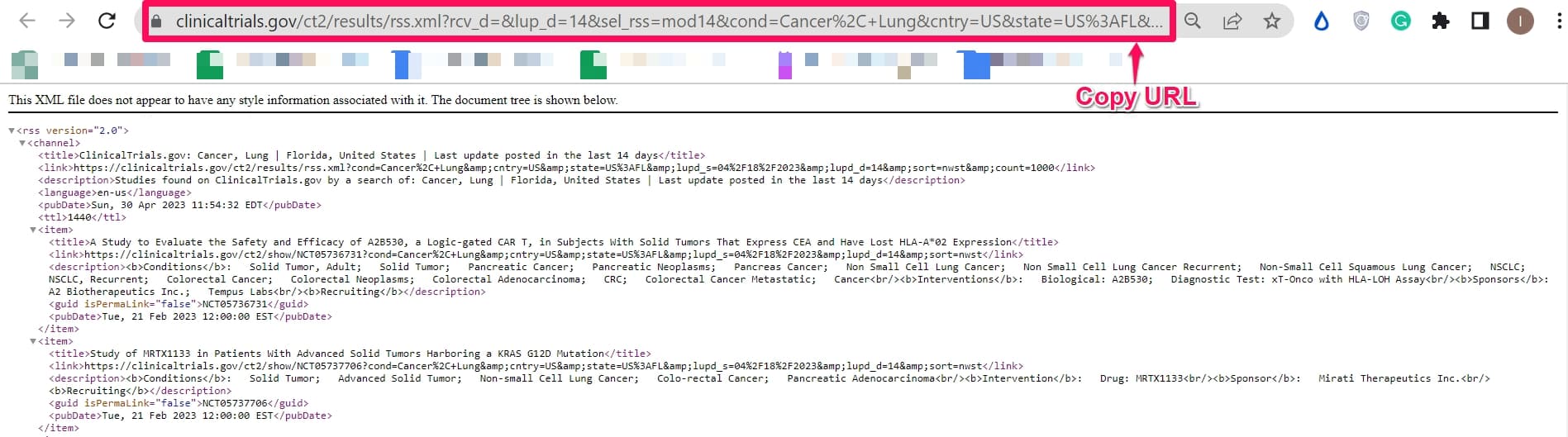
Step 6: Go to the Distill watchlist and click the arrow on the Add Webpage drop-down list.
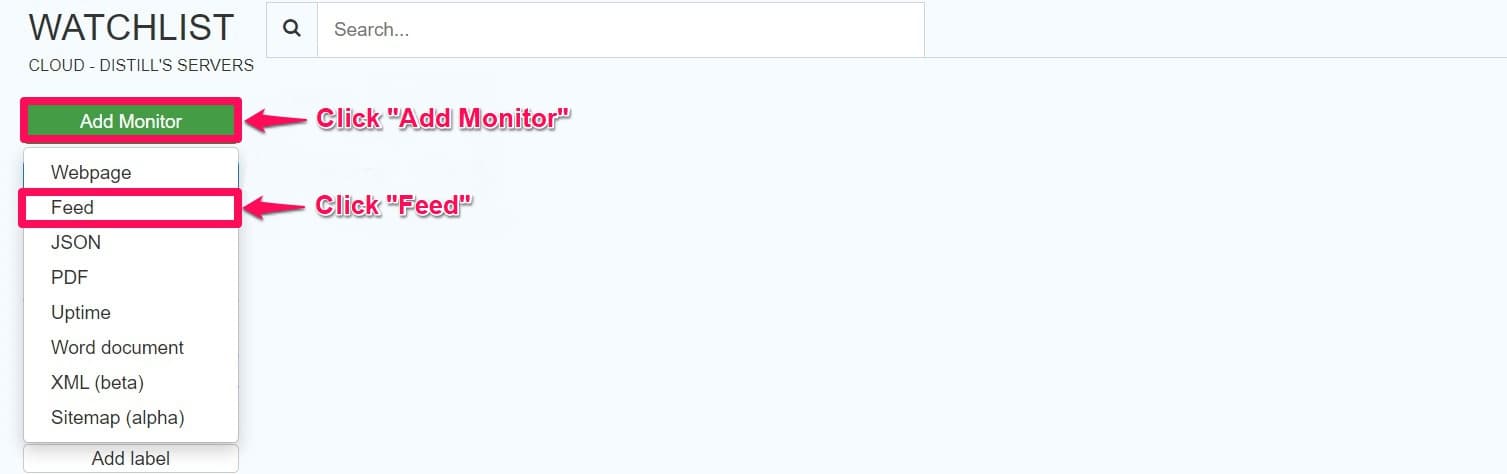
Step 7: Click Feed, paste the copied URL under GET, and click Go.
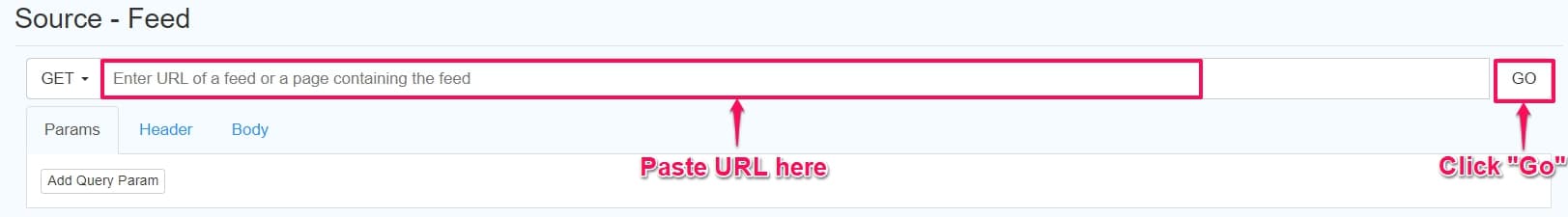
Step 8: Click Select proceed to the options page for the monitor.
Step 9: On the options page, you can set the device, alert type, and time interval for the monitor to track the feed.
Step 10: Click Save.
RSS Feeds are an easy way to stay up to date and avoid any false positives that may arise due to bad selections.
You can follow the steps given in this section to import and edit the JSON provided below.
Here is the JSON for monitoring the RSS feed of lung cancer studies in Florida on clinicaltrials.gov: Download Monitor JSON
How to track clinical trials backed by a particular sponsor
The clinical trial industry is highly competitive due to the numerous companies and organizations developing and testing new drugs and medical devices. Investors and sponsors often monitor their competitors for the following reasons:
-
Tracking new studies that they have invested in or sponsored.
-
Keeping a pulse on the various categories of studies that they are focusing on.
-
Checking trial progress to track any commercialization opportunities.
-
Review the reports and testing phases of the clinical trials conducted by competitors to find gaps or malpractices.
Follow the below steps to track your competitors on clinical registries:
Step 1: Navigate to clinicaltrials.gov and click the By Topic tab. Under this tab, go to the sponsor/collaborators option. You can select your competitor’s name in the list. For this example, we will be monitoring Abbott Medical Devices.
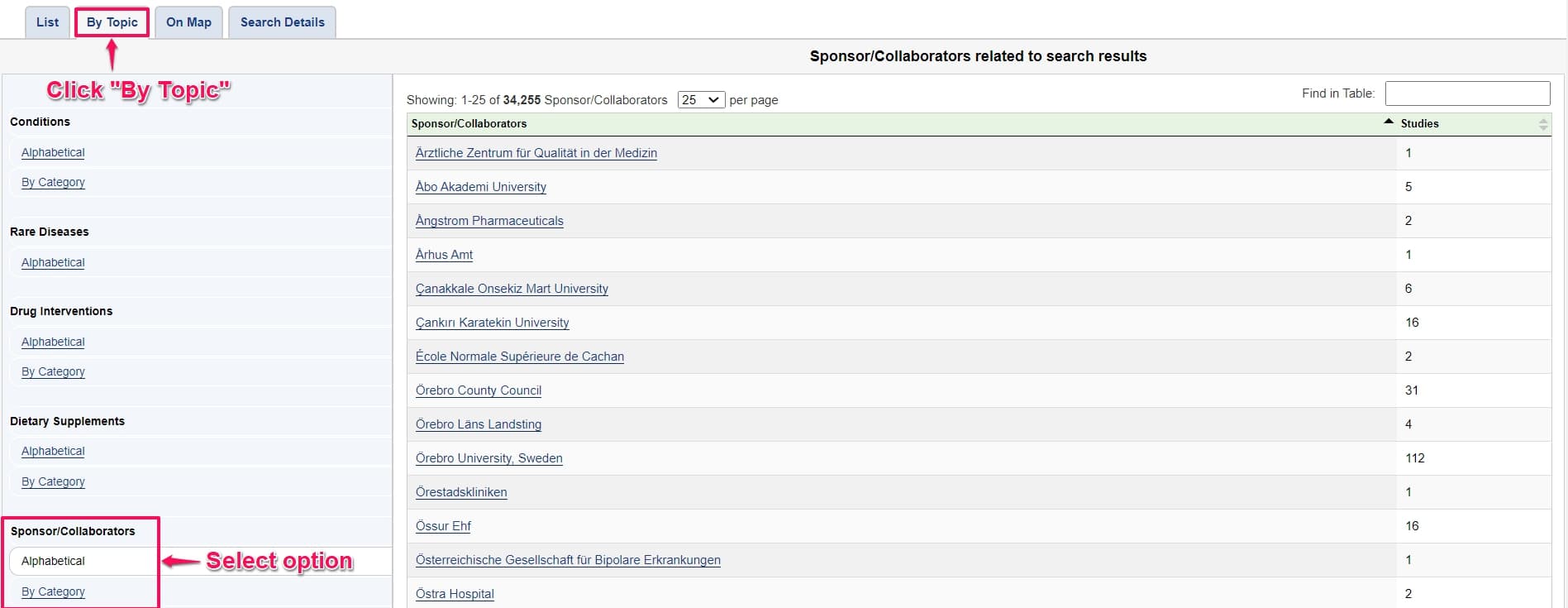
Step 2: Click the Distill icon on the top right corner of the webpage and click Monitor parts of page.
Step 3: To select the first record in the feed, click the serial number 1 and then choose the first trial record using the expand selection option. This will ensure you get an alert every time the feed is updated with a new trial record. Refer to the images in this section to learn more about how to set up this monitor.
Note: Make sure you have sorted the clinical trials by the Newest before setting up the monitor. For more information, refer to this section.
Step 4: Click Save selections.
Step 5: Once directed to the options page, choose a device to run your monitor. We suggest using your local browser if you use the free plan.
Step 6: Click Save.
How the monitor works
You’ll get instant alerts whenever the first row changes with new data related to your competitor’s activity. By keeping an eye on the competitor’s feed, you can easily discover any new clinical trials they are engaged in.
Once you receive an alert for a new clinical trial, you can set up a webpage monitor to track the status and results of the clinical trial. Refer to this section to learn how to set up monitors for individual trial web pages.
You can follow the steps given in this section to import and edit the JSON provided below.
Here is the JSON for monitoring trials backed by Abbott: Download Monitor JSON
How to track the recruitment status of a clinical trial
Monitoring the recruitment status of a clinical trial can be helpful if you want to:
-
Enroll quickly to participate in particular research relating to your clinical condition, to access treatment that is yet to be available to the general public.
-
Check if a trial is actively recruiting participants, if you have invested in or sponsored the research.
Follow the steps below to set up Distill monitors to automate the process of tracking the status of a clinical trial:
Step 1: Go to the clinical trial website. In this example, we will be tracking a study on BP control in stroke patients registered on clinicaltrials.gov.
Step 2: Click the Distill icon on the top-right corner of the webpage you are interested in tracking and click Monitor parts of page.
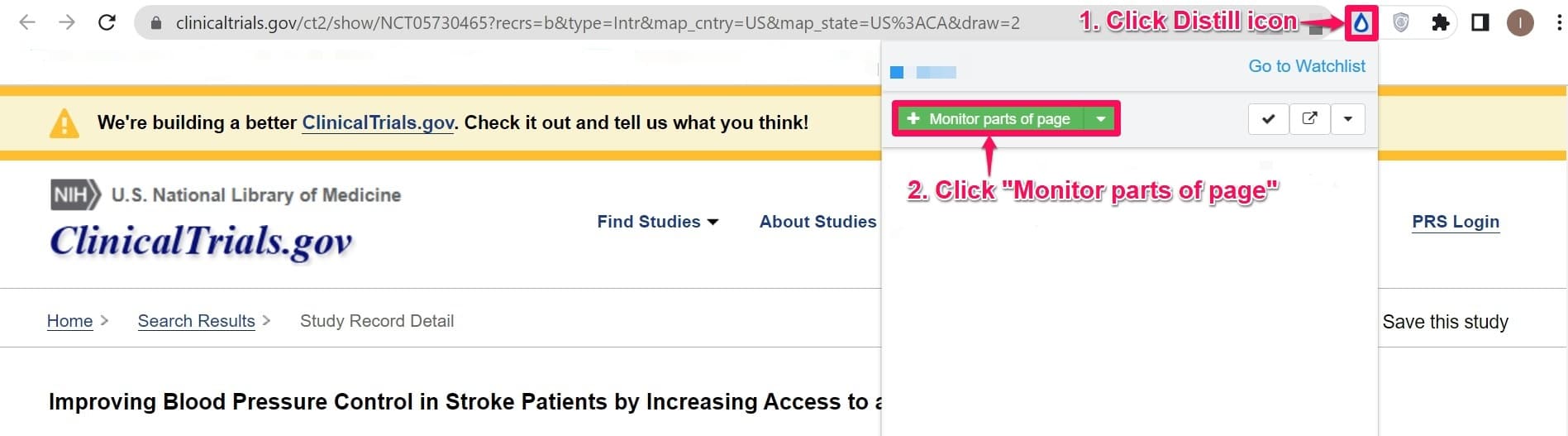
Step 3: Click the Recruitment status section of the webpage. Note that the current status of the clinical trial is Not recruiting.
Steps to refine selection:
Sometimes, you may be unable to select only the recruitment status from the status of the clinical trial section. Follow these simple steps to refine your selection:
-
Click the green tile, which will select the entire box.
-
Click the text within the section you don’t want to track. E.g., First posted, Last update posted, and See Contacts and Location subsections within this section.
-
The sections you clicked will be highlighted in red, indicating that you have deselected them.
-
Once done, Distill will only monitor the recruitment status on the clinical trial website. As this is the only section that is being tracked in the green tile.

Step 4: Click Save selections.
Step 5: On the options page, choose a device to run your monitor. We suggest using your local browser for checks if you are on a free plan. You can also select the schedule checks, to choose the time interval at which Distill will check the web page for updates.
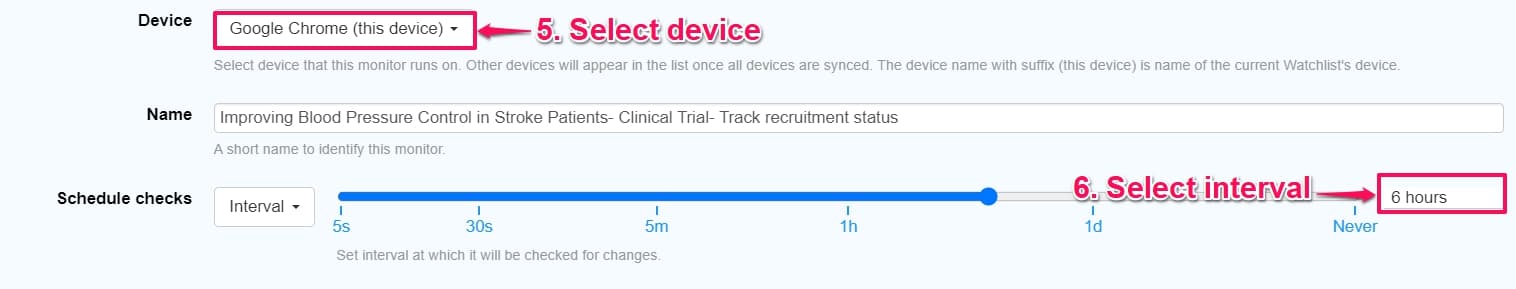
Step 6: Under the Action section, select how you want to be alerted. You can configure your alert mode via email/SMS/ Discord, etc.
Step 7: Click Save.
How the monitor works:
The Distill monitor will track the selected section every 6 hours (depending on what you have chosen). Once the clinical trial’s status changes, it will instantly send you an alert.
Once the clinical study begins recruiting volunteers, you can contact your local health consultant to learn more about the experiment and register for it.
You can follow the steps given in this section to import and edit the JSON provided below.
Here is the JSON for monitoring trial’s recruitment status for a trial on BP control in stroke patients: Download Monitor JSON
How to track updates on the trial and check if study results are posted
If you work as a regulator in the medical industry or are sponsoring trials, you might have to monitor the results tab of hundreds of clinical trial web pages. Some parameters you may want to track might include:
-
Trial’s expected end date.
-
Status of the trial and following the developments.
-
Outcomes of the clinical trials and discrepancies, if any.
-
Publications related to the research.
Follow the below steps to track the status of a clinical trial:
Step 1: Navigate to the clinicaltrials.gov. For this example, we will track a study to improve BP control in stroke patients.
Step 2: Go to the No results posted tab.
Step 3: Click the Distill browser extension icon on the top right corner of the webpage that you want to track and choose and click Monitor parts of page.
Step 4: Select the section under the No results posted tab. This section is a collection of trial updates.
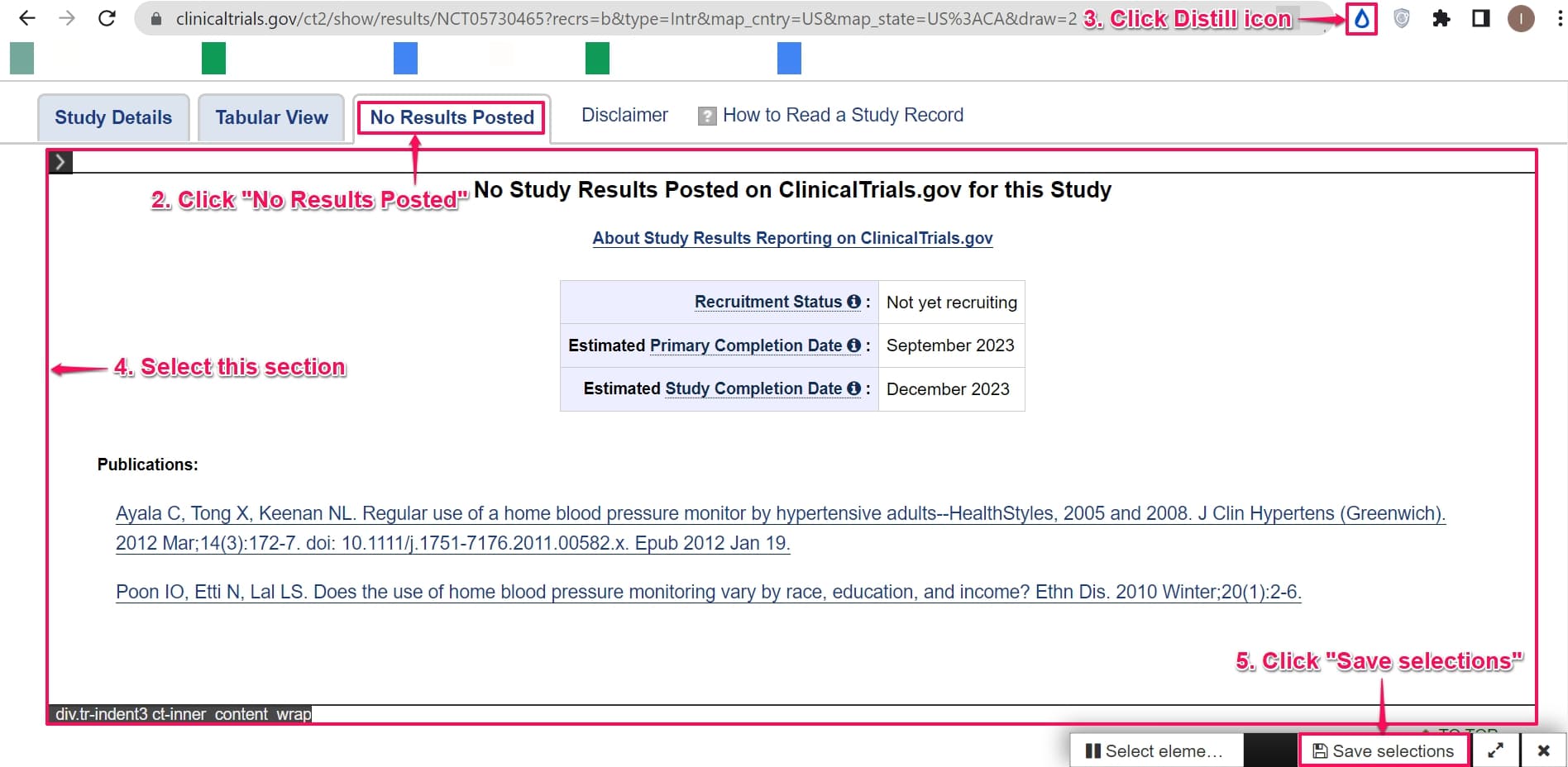
Step 5: Click Save selections.
Step 6: Choose a device to run your monitor. For faster and frequent checks, we recommend using your local browser.
Step 7: Click Add action to set up your notification mode. Note that on a free plan, you can access only 30 emails a month. We suggest using local push notifications for faster checks.
Note: If you need to track multiple clinical trials simultaneously, our subscription plans allow you to do so at scale and with custom frequency. You can also contact us to learn more about our enterprise plans.
Step 8: Click Save.
How the monitor works
You will be alerted when the results section changes anytime or has new updates. You can take appropriate action based on your use case when you receive an alert regarding changes to the section. For example, you’ll want to contact the sponsor responsible for the trial if the scheduled end date has been postponed.
You can follow the steps given in this section to import and edit the JSON provided below.
Here is the JSON for tracking study results for a trial on BP control in stroke patients: Download Monitor JSON
Steps to import and edit JSON
Steps to import with JSON
Once you’ve downloaded the JSON file from the previous section, you can easily import it into your watchlist.
To import the JSON file into Distill, you can simply copy the text and paste it into the “import JSON” section of the Distill browser extension, as shown in Distill docs.
Alternatively, you can also use the Distill webapp where you can use the Select JSON File option to import the downloaded JSON file.
You can also refer to this video to learn more about importing a monitor using JSON.
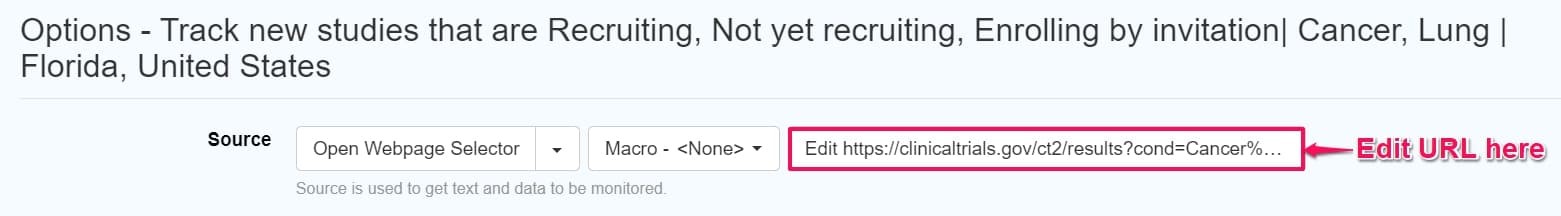
Steps to edit a monitor imported with JSON
To modify the downloaded/recorded JSON in Distill, simply click the down arrow on the monitor in the Distill watchlist and choose edit options. This will take you to the options page where you can paste the desired URL into the edit option under the source field.
Save the changes you make in the options page, and you’re all set to track edited monitor.
Note: This monitor will work as long as webpage structure and UI remains similar.
Conclusion
Distill can be an effective tool for tracking clinical trials. With it, you can stay up to date on the latest developments related to the trial, including:
-
Details about its publications and reports.
-
Volunteer recruitment status.
-
Gain insights into the trial’s progress over time.
-
Organize and track multiple clinical trials with labels and view their updates from a single dashboard.
You can download the Distill extension for Chrome, Firefox, Opera, and Microsoft Edge.
Check out the Distill subscription plans to access additional monitors for checking multiple trial websites simultaneously. The subscription also comes with features such as push notifications on iOS and Android apps.
You can contact us on Distill forums if you have any queries while setting up your trackers. Here we have an active community of users who will lend a hand and sort out your problem quickly.
You can also contact the Distill support team to learn more about our enterprise plans.
 Distill
Distill